-
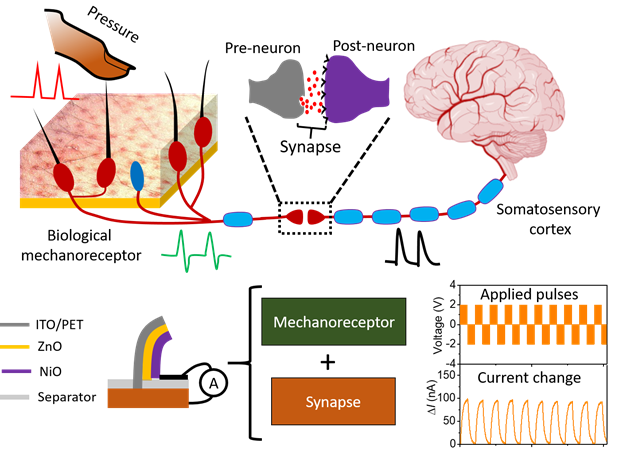
A team of researchers led by Professor Seo Hyeong-tak of Ajou University has developed an artificial synapse for tactile perception. The new device, which emulates tactile perceptions involving touch and nerves, features a hetero-junction of oxides. Expected applications include skin-attached electronics and artificial intelligence (AI) sensors.Prof. Seo (Dept. of Materials Science and Engineering / Graduate Dept. of Energy Systems, pictured) and his team’s work was published under the title, “An artificial piezotronics synapse for tactile perception,” in an online issue of Nano Energy on April 15, 2020. Also on the team from Ajou University were Prof. Kim Sang-wan (Dept. of Electrical and Computer Engineering) and Dr. Mohit Kumar (researcher, Dept. of Materials Science and Engineering). Of the five senses humans have at their disposal, tactile perception involves the signals stimulated by a touch traveling from the stimulus receptor at the terminal of the skin to cranial nerves via the neural network. Humans are able to discern an incredibly wide range of touches on their skin. Combined with emotions of varying depths, these tactile perceptions can become part of human memory.An artificial synapse that emulates the human tactile perception therefore epitomizes a highly sophisticated technology capable of detecting the wide variety of touches exerted from the outside world with finesse and precision, saving perceptions thereof, and retrieving them in a memory-like fashion.Prof. Seo’s team achieved such an advanced artificial synapse, featuring a hetero-junction of nickel and zinc oxides (NiO and ZnO) that emulates tactile stimulation of nerves. Specifically, the synapse was designed to emulate how hair detects changes in external pressure using a vertically structured sensor featuring the hetero-junction of oxides. It is this sensor that produces changes in the current-voltage curve in response to changing intensity and directions of pressure in a fashion similar to how the human body perceives touch.
-
5
- 작성자OI***
- 작성일2020-08-27
- 7199
- 동영상동영상
-
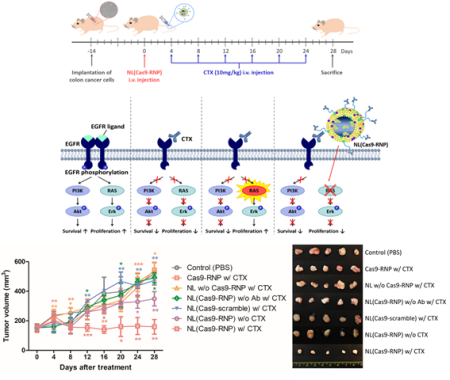
A team of Ajou University researchers led by Prof. Yoon Tae-jong has succeeded in developing a treatment for colorectal cancer using gene editing. The cure is specifically meant for colorectal cancer patients with gene mutations on whom other more common therapies do not work.Prof. Yoon (College of Pharmacy) teamed up with Prof. Cho Young-seok of the Catholic University (Department of Internal Medicine, College of Medicine) to develop a gene editing technique that involves mounting a gene-editing structure onto a nano-size carrier to edit genetic mutations that complicate colorectal cancer treatment. The result of their collaboration was published in an online issue of Nano Research, a widely recognized international journal, on April 4, 2020, with the title, “Gene editing particle system as a therapeutic approach for drug-resistant colorectal cancer.”Cetuximab is a widely used treatment for colorectal cancer, but there are also a significant number of patients whose KRAS gene mutation renders them resistant to the drug.The number of patients newly diagnosed with colorectal cancer is on the rise worldwide due to the increase in modern and unhealthy diets. Finding effective cures to this particular cancer is therefore a popular and important topic of pharmaceutical research, with much of the current research focusing on antibodies. The conventional approach to treatment is to introduce a drug that binds to the EGFR receptor to inhibit the growth and survival of cancerous cells. Nearly 60 percent of colorectal cancer patients, however, are genetically resistant to this type of treatment. The prognosis for these patients with the KRAS gene mutation is rather dim.Although a growing number of researchers focus on finding alternative cures for patients with the KRAS gene mutation, that particular genetic mutation facilitates patient relapse, raising the demand for a more fundamental therapeutic approach. Although scientists have speculated that gene editing could pave the way to a useful alternative, they have been struggling to overcome the resulting inefficiency, as these protein-based genetic editing techniques turn quite volatile in vivo. Prof. Yoon’s team thus introduced this breakthrough, whereby they demonstrated that mounting the gene-editing protein onto a nano-size carrier could effectively treat colorectal cancer. The team applied a nano-liposome structure as a carrier for the gene-editing technique that can get to and remove the mutated KRAS gene. By inserting a cancer-targeting antibody into the surface of this nano-structure, the researchers were able to maximize stability and efficacy of the gene editing. In an in-vivo test, the gene-editing structure had an efficiency of 60 percent in targeting cancerous tissues. The technique had a definitively more therapeutic effect on targeted animals as compared to the control group.Prof. Yoon explained: “Existing gene editing techniques rely on viral carriers that require adaptation in vivo before their effects appear. This meant low efficiency and increased risk of off-target editing. Although protein-based gene editing techniques have emerged as an alternative, the fact that these protein carriers were broken down in vivo by enzymes meant the targeting efficiency was inevitably compromised as well.”His team therefore came up with a nano-structure capable of in-vivo adaptation to enhance gene editing stability and efficiency, thereby significantly improving the overall therapeutic value of the gene editing technology in cancer treatment.Prof. Yoon commented: “At present, gene editing in the context of cancer treatment is mostly used to regulate the activity of immune cells in vitro. With our discovery, we expect the medical community to be able to inject gene-editing structures directly into patients’ bodies to maximize the effect of treatment.”The KRAS gene mutation accounts for complications in treatment beyond colorectal cancer, to include treatment for pancreatic, lung, ovarian and other types of cancer. Nano-gene editing is therefore desperately needed to enable cancer patients to receive the life-saving treatment they need.Prof. Yoon has been researching nano-materials capable of effectively delivering diverse biomaterials into cells and tissues for the last two decades. In recent years, he has concentrated on adapting nano-technology to gene editing so as to enhance the latter’s in-vivo stability and cell permeability and also to facilitate localized treatment. Prof. Yoon is also the CEO of Moogene Medi Inc., part of N4U Tech Holdings Inc., which leads Ajou University’s industrial-academic collaboration programs. He intends to have Moogene Medi organize the clinical trials for his team’s groundbreaking discovery.
-
3
- 작성자OI***
- 작성일2020-08-27
- 6930
- 동영상동영상
-
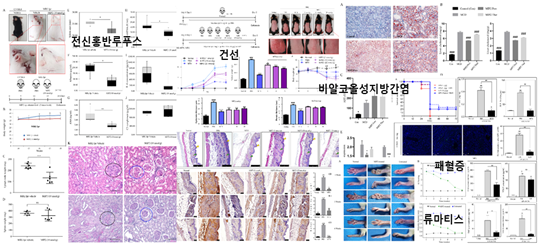
A team of researchers at Ajou University, including Professors Choi Sang-dun and Kim Wook, has found a cure for several autoimmune inflammatory diseases. The team has successfully demonstrated the efficacy of their product in treating diseases like systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA).The team’s groundbreaking paper was published in an online issue of Biomaterials, an international journal, on March 17, 2020. Professor Choi (Dept. of Molecular Science and Technology, Graduate Dept. of Life Sciences) led the work as the corresponding author, with participation from other Ajou faculty members, including Profs. Kim Wook (Molecular Science and Technology), Suh Chang-hee (Dept. of Rheumatology, School of Medicine), and Kim Soon-sun and Cheong Jae-youn (both Dept. of Gastroenterology, School of Medicine). Research Professor Masaud Shah and Prof. Lee Sang-ho (Division of Nephrology, Department of Internal Medicine at Kyung Hee University Hospital at Gangdong) also participated. Kim Gi-young and Asma Achek, both studying molecular science and technology at Ajou, were also listed as co-principal investigators.The researchers developed a peptide-based treatment for autoimmune inflammatory diseases, and demonstrated its efficacy in treating non-alcoholic steatohepatitis (NASH), SLE, RA, eczema and sepsis in animals.An autoimmune disease is an abnormal immune response of the body to its own tissues or cells. There are approximately 80 autoimmune diseases that have been identified so far. Inflammatory diseases refer to a broad array of diseases in which inflammation is a major symptom, and can include NASH, sepsis, and even Alzheimer’s.There are mainly two mechanisms by which autoimmune and inflammatory diseases occur: either in response to an infection by an outside pathogen or as a non-infectious response to substances generated within the immune host itself. Abnormal activation of the Toll-like receptors (TLRs) is involved in both cases, but not much more is known. The Ajou team of researchers has discovered a peptide (MIP2) that can inhibit the activity of TLRs across a wide range of situations to rein in inflammatory cytokines.Prof. Choi explained: “We have discovered that injecting this peptide, MIP2, into test models cured them of NASH, SLE, RA, eczema, and sepsis. In addition, we are researching and testing the effect of this peptide on inhibiting inflammasomes and thereby possibly treating other inflammatory diseases, including Alzheimer’s, type-II diabetes, and multiple sclerosis.”The project was organized with support from the R&D-Centered Hospital Support Program of the Ministry of Health and Welfare, as well as the Basic and Original Research Support Program, co-sponsored by the Ministry of Science and ICT and the National Research Foundation of Korea.
-
1
- 작성자OI***
- 작성일2020-08-27
- 4415
- 동영상동영상
-
Ajou University Professor Sang Dun Choi developed a medicine for Systemic Lupus Erythematosus, an autoimmune disease in which the body's immune system mistakenly attacks healthy tissue in many parts of the body.The research team led by Professor Choi (Department of Biological Science, Graduate School's Department of Molecular Science and Technology, pictured above) said that it succeeded in deriving an innovative therapeutic substance following the verification of its remedial effect in the lupus-related murine model.A paper on the findings was published in the February edition of The EBioMedicine, which ranks within the top 7.7 percent among medical journals, under the title "A peptide derived from the core β-sheet region of TIRAP decoys TLR4 and reduces inflammatory and autoimmune symptoms in murine models."Systemic Lupus Erythematosus involves various symptoms such as skin rashes, photosensitivity, arthritis, canker sores, nephritis, hematocytopenia, panangiitis, and serositis. It is known to occur in those with genetic predispositions when they are infected with certain viruses or influenced by environmental factors like ultraviolet rays.In Korea, the number of lupus patients has been increasing from 20,902 in 2015 to 26,556 in 2019 and more than 5 million people are known to suffer from lupus around the world. In most cases, women in their 20s and 30s fall victim to lupus.The team led by Professor Choi identified a toll-like receptor, which is activated by an intrinsic damage-associated molecular pattern, as a factor accelerating the autoimmunocyte reaction and verified the therapeutic effect of the TLR7/9-controlling substance in murine models.The research was conducted with the support of the Original Technologies Development Project carried out by the Ministry of Science and ICT and the National Research Foundation of Korea. Professor Chang Hee Suh (School of Medicine) and Professor Wook Kim (Graduate School's Department of Molecular Science and Technology) from Ajou University and Professor Dae Hyun Hahm from Kyung Hee University School of Medicine also participated in the research, and the findings are being promoted by the National Research Foundation of Korea as a research achievement that provides direct benefits to the public.Professor Choi said, "Medicines that are based on TLR control can be applied to various other autoimmune and inflammatory diseases once their effect is proven for a single autoimmune disease," adding, "Therefore, our findings are highly likely to be developed into a blockbuster medicine."
-
-1
- 작성자OI***
- 작성일2020-08-27
- 4527
- 동영상동영상
-
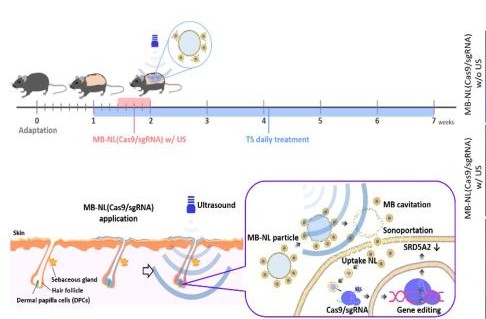
Professor Yoon Tae-jong and his team in the College of Pharmacy have found a genome-editing technology that can be used to treat androgenic alopecia. This latest discovery is raising expectations for finally finding an alopecia treatment with minimal side effects and maximized efficacy. Professor Yoon (pictured) has announced his finding that an ultrasound-reactive nano-level genome-editing technology and delivery system can offer a more effective androgenic alopecia therapy. The discovery was published in the online issue of Biomaterials, a noted academic journal, on December 28, 2019, under the title, “Ultrasound-activated particles as CRISPR/Cas9 delivery system for androgenic alopecia therapy.” Androgenic alopecia occurs when testosterone is converted by a reductase known as SRD5A2, found in hair follicle cells on the scalp, into dihydrotestosterone (DHT), which then serves to inhibit the growth of those cells. As such, it differs from alopecia caused by environmental factors, such as stress and smoking. The reductase that is responsible for DHT is mostly concentrated on the front and crown of the head. It is the genetic overexpression of this reductase that causes the loss of hair from those parts of the scalp. Alopecia due to environmental factors, on the other hand, can affect any part or the entirety of the scalp. As hereditary/hormonal alopecia can cause significant distress to patients and compromise their quality of life, many researchers worldwide have been exploring pharmaceutical treatments. The existing treatments for androgenic alopecia, such as dutasteride and finasteride, are oral reductase inhibitors that affect reductases in not only the scalp but also other organs, leading to a host of side effects, including hypotension, gynecomastia (breast enlargement in men), liver toxicity, and erectile dysfunction. These side effects make it impossible for patients to stay on these pills for extensive periods of time. Professor Yoon and his team have found that the genome-editing technology known as CRISPR-Cas9 (sgRNA), using nanobubbles of air that react only to stimuli from external ultrasonic sources, is highly effective in delivering alopecia treatments to parts of the scalp that conventional nanostructures cannot reach easily. Specifically, the team has developed a genome-editing technology for applying an ointment-type, rather than pill-type, alopecia treatment. After inducing androgenic alopecia in test animals, the researchers spread an ointment-type treatment, containing liposome nanobubbles with a protein-type genome-editing molecule, on the animals’ skin and subjected it to ultrasound waves. They discovered that the method was highly effective. Furthermore, the researchers found that the genome-editing technology they employed targeted and destroyed only SRD5A2 to inhibit its growth in hair follicle cells only. This discovery has the potential to lead to the development of an enduring treatment for alopecia that can be spread on patients’ scalp. The treatment method did not generate any of the side effects associated with conventional alopecia treatments. In fact, it led to hair growth in only eight weeks. “The most important achievement of our study,” Professor Yoon emphasized, “is our finding that simple ultrasound stimulation can deliver a protein-type genome editor into otherwise impenetrable hair follicle cells with great efficiency.” He went on, “Although genome-editing technology has been expected to provide a fundamental cure for alopecia, researchers have, until now, been struggling to adapt it to nano-level use.” Professor Yoon also commented, “By combining this nanotechnology with genome editing, we may be able to minimize the side effects and maximize the efficacy of alopecia therapy. The infinite potential of genome editing can greatly enhance the delivery of conventional pharmaceutical treatments for treat intractable and rare diseases.” For the last two decades, Professor Yoon has been researching a wide variety of nano-techniques for delivering various biocompounds into cells and tissues. In recent years, he has been focusing on adapting nano-techniques to genome editing with the aim of enhancing the in-vivo stability and cellular permeability of therapeutic substances and enable localized genome-editing treatments.
-
-3
- 작성자통합 관리자
- 작성일2020-03-08
- 6012
- 동영상동영상